© ROY SCOTT/GETTY IMAGES
© ROY SCOTT/GETTY IMAGES
In a dark room in Charlottesville, Virginia, a mouse swims in a small pool, searching for a place to rest. In 12 previous swims, with the help of visual cues and training from an experimenter, the mouse eventually tracked down a platform near the center of the pool. But just a day after its last swim, the animal is spending nearly as much time searching for the platform as it did on its first swim. The discombobulated mouse’s problem? It has no T cells.
“Mice without functional T cells do not perform cognitive tasks as well as wild-type mice do,” says the University of Virginia’s Jonathan Kipnis, who first demonstrated a link between the immune system and cognitive function in 2004 as a member of Michal Schwartz’s lab at the Weizmann Institute in Rehovot, Israel.1 He later discovered that T cells’ pro-cognitive...
Kipnis’s work is part of a wave of research changing the way scientists view the relationship between the immune system and the central nervous system (CNS). Until recently, the brain and the spinal cord were considered immune-privileged sites, strictly cordoned off from immune cells unless something went terribly wrong. Researchers knew, for example, that multiple sclerosis (MS) was caused by T cells that breach the selective border called the blood-brain barrier (BBB), enter the CNS, and attack the myelin sheath covering neurons. Even microglia, specialized macrophage-like immune cells that scientists had recognized as normal CNS residents since the 1960s, were mainly studied in the context of disease.
Saying the immune system is always good for the brain, it’s wrong; saying it’s always bad for the brain, it’s wrong. It depends on the conditions.—Jonathan Kipnis,
University of Virginia
But over the past two decades, researchers have recognized that the entire immune system is very much a part of a functional CNS, with vital roles in cognition, injury repair, neurodegenerative disease, and sensory systems. Microglia pervade the CNS, including the white and gray matter that constitute the organ’s parenchyma. Other immune cells, including T cells, monocytes, and mast cells, reside in the brain and spinal cord’s outer membranes, known as the meninges, and circulate in cerebrospinal fluid (CSF).
“Ten or 15 years ago, it was all bad,” Serge Rivest, a neuroscientist at Québec’s Centre Hospitalier de l’Université Laval (CHUL), says of the relationship between the brain and immune cells. These days, he says, researchers are focused on understanding the good in addition to the bad and the ugly.
Injury patrol
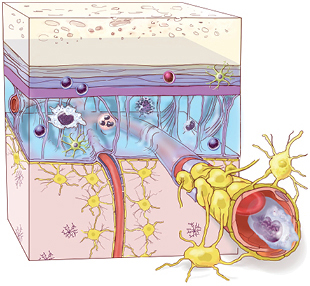 IMMUNITY IN THE BRAIN: Until recently, the central nervous system was thought to be cordoned off from the peripheral immune system, but researchers now know that diverse immune cells—possibly by the millions—circulate in the cerebral spinal fluid and live in the brain’s outer membranes even in healthy individuals.
IMMUNITY IN THE BRAIN: Until recently, the central nervous system was thought to be cordoned off from the peripheral immune system, but researchers now know that diverse immune cells—possibly by the millions—circulate in the cerebral spinal fluid and live in the brain’s outer membranes even in healthy individuals.
See full infographic: WEB | PDF© 2016 TERESE WINSLOW LLCAs early as the 1980s, researchers knew that immune cells infiltrated the CNS after injury, but such immune activity was viewed as something to be stymied, not encouraged. In fact, doctors used corticosteroids, which suppress immune-cell activity, to treat brain injuries for many decades. But Schwartz says it didn’t make sense to her that tissues as indispensable as the brain and spinal cord wouldn’t take advantage of the immune system’s ability to protect against pathogens and repair damaged tissues. In the mid-1990s, she began searching for a positive neurological role of the immune system.
After nicking the spinal cords of rats, her team demonstrated that injecting macrophages at the injury site restored the animals’ motor function. The macrophages facilitated healing, as they are known to do in other tissues such as liver and muscle.3 (See “Immune Cell–Stem Cell Cooperation,” The Scientist, July 2016.) Around the same time, other researchers were finding that eliminating macrophages improved recovery from spinal cord injury in mice and rats.4 Because of this, Schwartz recalls, her work “was met with a high degree of skepticism.”
But over the next decade, Schwartz and others continued to unveil more ways that the immune system promotes CNS repair after trauma. Macrophages, for example, can damage neurons by secreting cytokines, proteases, or reactive oxygen species, but in rat and mouse models of spinal cord injury, they also produce transforming growth factor-beta (TGFβ), which promotes wound healing,5 and interleukin 10 (IL-10) which helps resolve inflammation.6 By the late 2000s, researchers recognized that different subtypes of macrophages can benefit neuronal growth in rodents, and that some were critical to recovery.7 Views also began to change on the clinical side after the 2004 Corticosteroid Randomization After Significant Head Injury (CRASH) study showed that corticosteroids didn’t help brain injury patients recover, but increased their risk of disability and death.8
More recently, research has revealed that it’s not just macrophages and other components of the innate immune system that help maintain CNS health; cellular drivers of adaptive immunity also contribute. In 2013, Schwartz and her colleagues demonstrated in mice that the lining of each of the brain’s four ventricles harbors memory T cells whose receptors bind proteins found in the CNS.9 Although these T cells are specific for CNS proteins, they don’t cause autoimmune disease. Schwartz contends that their specificity allows them to respond to local CNS damage.
Her team also showed that T cells present in this lining, called the choroid plexus, secrete cytokines such as interferon gamma (IFNγ), which allows selective passage of CD4+ T cells and monocytes from the blood into CSF within the ventricles.10 In a model of spinal cord bruising, mice deficient for the IFNγ receptor had reduced immune cell trafficking across the choroid plexus and poor recovery of limb movement. And last year, Kipnis’s team reported that IL-4 produced by CD4+ T cells in the CNS signals neurons to regrow axons after spinal cord or optic nerve injury.11
 IN SICKNESS AND IN HEALTH: The immune system is a critical part of a healthy central nervous system (CNS), as well as the CNS's response to injury.
IN SICKNESS AND IN HEALTH: The immune system is a critical part of a healthy central nervous system (CNS), as well as the CNS's response to injury.
See full infographic: WEB | PDF© 2016 TERESE WINSLOW LLCTo better understand how different immune cells contribute to injury repair, Dorian McGavern, an immunologist at the National Institute of Neurological Disorders and Stroke in Bethesda, Maryland, is tracking responses to CNS injury in real time. Using two-photon microscopy to image cells below the surface of brain tissue in living animals, he and his colleagues have found that minutes after injury, microglia clear up debris in the parenchyma. “They’ll look around the environment, and basically start street-sweeping and picking up all the dead material,” McGavern says. Meanwhile, other immune cells remain confined to the meninges and to the so-called perivascular spaces between larger cerebral blood vessels and their sheaths of pia mater, the innermost meningeal membrane.
“When we watch the anatomy [after injury], we see that the microglia will stay in the brain parenchyma, and the neutrophils and macrophages will come to the lining of the brain and the perivascular spaces,” says McGavern.
When he and his colleagues prevented macrophages from entering the CNS by blocking receptors that respond to nearby cell damage, mice fared worse within the first 24 hours after injury.12 “In every case, we have created more injury and more [neural] cell death.” His team also found that microglia reinforce the BBB, which is composed of endothelial cells, pericytes, and astrocytes. Microglia fill in spaces left by astrocytes killed or damaged during injury. Without a robust barrier, McGavern says, unwanted immune cells may flood the parenchyma and do more harm than good.
Balancing health and disease
When Rivest first presented his work at a meeting, practically the whole audience lined up behind the microphone to make skeptical comments, he recalls. “I was really not well received there.” He adds that he himself was an early skeptic. “At the beginning, we were convinced that very strongly activated [immune] cells were bad for the brain, [but] it turned out that [they] prevent circulation in the brain of proteins that are neurotoxic.”
 HUNGRY PHAGOCYTES: Monocytes (green) from the blood are attracted to amyloid deposits (red) in cortical veins of the murine brain. These cells help clear the plaques, improving the mice’s cognitive performance.CELL REPORTS, 5:646-53, 2013Looking beyond immune cells’ negative roles in neurological diseases has led researchers to some unexpected immune functions in the CNS, including the role for T cells in learning and memory that Kipnis described. Initially, he and his colleagues observed that mice without T cells are slower to learn in a water maze-based test of memory.1 The researchers could restore normal cognitive abilities to these mice by injecting them with wild-type T cells.20
HUNGRY PHAGOCYTES: Monocytes (green) from the blood are attracted to amyloid deposits (red) in cortical veins of the murine brain. These cells help clear the plaques, improving the mice’s cognitive performance.CELL REPORTS, 5:646-53, 2013Looking beyond immune cells’ negative roles in neurological diseases has led researchers to some unexpected immune functions in the CNS, including the role for T cells in learning and memory that Kipnis described. Initially, he and his colleagues observed that mice without T cells are slower to learn in a water maze-based test of memory.1 The researchers could restore normal cognitive abilities to these mice by injecting them with wild-type T cells.20
In 2013, Rivest used two-photon microscopy to monitor monocytes in blood vessels of living mouse brains, and he watched as the cells migrated toward and cleared amyloid-β deposits within veins. When the researchers selectively depleted monocytes, the mice developed more amyloid-β plaques in the cortex and hippocampus.14 And when they knocked out the innate immune signaling protein MyD88, which mediates signals from several monocyte-activating receptors, the mice also experienced more amyloid-β accumulation, accompanied by accelerated cognitive decline.15
More recently, Rivest’s team found that microglia-forming monocytes are beneficial in a model of MS, where microglia are found within the inflammatory lesions. Last year, the researchers reported that inhibiting monocytes from entering the CNS reduced the clearance of damaged myelin and impeded proper remyelination.16
Schwartz has similarly found evidence for the immune system’s ability to protect against neurodegeneration. Last year, she and her colleagues reported that the choroid plexus epithelium was less permissive to immune cell trafficking in a mouse model of Alzheimer’s disease than in wild-type mice, due to anti-inflammatory signals produced by regulatory T cells (Tregs). They found that depleting Tregs in Alzheimer’s mice allowed macrophages and CD4+ T cells into the brain, reduced the number of amyloid-β plaques, and improved cognition.17 Similarly, blocking the T-cell checkpoint protein PD1, which normally supports Treg survival while suppressing the activity of other T cells, reduced amyloid-β plaques in mouse brains and improved the animals’ scores in a learning and memory water maze test.18
In 1996, Schwartz cofounded Proneuron Biotechnologies, which plans to test antibodies that target PD-1 in Alzheimer’s patients. This, she says, would be the first proinflammatory approach to treating a neurodegenerative disease, where immune activation has long been seen as a contributor to neural damage.
In addition to repairing neural injury, immune cells appear to play a role in fighting neurodegenerative disease.
But there’s a reason that scientists have believed that immune activity contributes to Alzheimer’s damage: microglia, perhaps best known for trimming back synapses, have the potential to become overzealous, and excessive synapse pruning can cause neural damage in a variety of CNS diseases. By blocking the cells’ proliferation in mice, Diego Gomez-Nicola of the University of Southampton in the U.K. has successfully alleviated symptoms of Alzheimer’s disease, amyotrophic lateral sclerosis, and prion disease. And earlier this year, Beth Stevens of the Broad Institute and her colleagues reported that inhibiting a protein that tags synapses for microglial pruning halted over-pruning and loss of synapse signaling strength in two mouse models of Alzheimer’s disease.19
“You’ll probably find just as many papers saying that microglia are good as microglia are bad,” says Gomez-Nicola, “and neither one nor the other is true.”
Rivest says a fuller appreciation of the benefits of immunity in the CNS could open a lot more doors for potential treatments than simply looking for ways to block inflammation whole hog. “The field is really moving toward that direction,” he says.
Behavior modification
Kipnis says regulation of stress may be linked to T cells’ role in learning. Stress can signal macrophages to secrete proinflammatory cytokines, some of which block a protein called brain-derived neurotrophic factor (BDNF), which astrocytes need to support learning and memory. CD4+ T cells in the meninges make more IL-4 cytokine after mice have been trained in a water maze—a stressful exercise for the animals—suggesting the signaling molecule might let macrophages know when the brain is dealing with the stress of learning something new, not the stress of an infection. “They tell macrophages, ‘Don’t overshoot,’” says Kipnis. In mice whose meninges are depleted of CD4+ T cells and thus deficient for IL-4, macrophages secrete proinflammatory factors unchecked in times of stress, disrupting their ability to learn and form memories.2
Last July, Kipnis and his colleagues also reported that mice lacking B and T cells were less social: while control mice spent more time investigating other mice than inanimate objects, immune-deficient mice had no preference.21 The researchers observed the same behavior shift in immunocompetent mice when they blocked a protein on T cells that facilitates migration to the CNS, or when they knocked out IFNγ, which Schwartz’s work has shown facilitates immune-cell migration through the choroid plexus.
 HELP OR HARM: Mast cells (shown here) and other immune cells can support the health of the central nervous system and aid in injury repair and pathogen defense, but overactivation can lead to neural damage.© CNRI/SCIENCE SOURCEKipnis speculates about an evolutionary link between immunity and social behavior; IFNγ both encourages social activity and protects animals from many communicable diseases. He and his colleagues observed that IFNγ levels are highest in the brain tissue of social animals, such as rodents and zebrafish, when the animals are wild or housed in captivity together rather than individually, suggesting that social interaction and T-cell immunity in the CNS reinforce each other.
HELP OR HARM: Mast cells (shown here) and other immune cells can support the health of the central nervous system and aid in injury repair and pathogen defense, but overactivation can lead to neural damage.© CNRI/SCIENCE SOURCEKipnis speculates about an evolutionary link between immunity and social behavior; IFNγ both encourages social activity and protects animals from many communicable diseases. He and his colleagues observed that IFNγ levels are highest in the brain tissue of social animals, such as rodents and zebrafish, when the animals are wild or housed in captivity together rather than individually, suggesting that social interaction and T-cell immunity in the CNS reinforce each other.
Others have proposed a link between behavior and innate immune cells called mast cells. Best known for their involvement in allergic responses in the upper airway, skin, and gastrointestinal tract, mast cells have been found in the meninges as well as in perivascular spaces of the thalamus, hypothalamus, and amygdala. They are known to quickly recruit large numbers of other immune cell types to sites of inflammation, and to play a role in MS. But mast cells also release serotonin into the hippocampus, where the molecule aids neurogenesis, supports learning and memory, and regulates anxiety.
Mice deficient in mast cells display deficits in hippocampal neurogenesis as well as in spatial learning.22 The animals also appeared more anxious, taking more time to enter an open space, for example.23 And Tufts University pharmacologist Theoharis Theoharides has found that human mast cells in culture respond to stress signals by releasing a growth factor that increases blood vessel permeability.24
Thus, like microglia, mast cells are a double-edged sword when it comes to neural health. It’s a reflection of the entire immune system’s love-hate relationship with the CNS, Kipnis says. “Saying the immune system is always good for the brain, it’s wrong; saying it’s always bad for the brain, it’s wrong. It depends on the conditions.”
Amanda B. Keener is a freelance science writer living in Winston-Salem, North Carolina.
MICROBIOME MEDIATION
The work suggested a role for gut microbes in neuroinflammation. But like so many components of the immune system itself, the microbiome’s relationship with CNS health goes both ways. Since his early studies, Kasper has found that resident gut microbes can program immune cells to protect the nervous system against immune overactivation. In 2010, his group showed that a gut bacteria–made sugar called polysaccharide A (PSA) activates a particular subset of Tregs that are induced by most MS treatments (J Immunol, 185:4101-08, 2010). Several studies have reported alterations in MS patient gut microbiota, though it’s not clear whether those changes are a result or cause of disease. “You have this balance going on in the gut,” Kasper says. “As long as everything is in balance, it’s homeostatic and everybody’s happy. But something happens in the [MS] disease process that that balance is lost.” The gut microbiome’s influence on the immune system has also been documented in mouse models of stroke, and appears to have similarly conflicting consequences. A study published last year showed that disrupting the gut microbial community with antibiotics reduced T-cell trafficking to the brains of mice after induced strokes, reducing neuronal damage (Nat Med, 22:516-23, 2016), for example, while another study indicated that a healthy gut microbiome is an asset for stroke recovery (J Neurosci, 36:7428-40, 2016). The authors of the latter study suggested that whether Tregs or proinflammatory T cells migrate from the gut to the brain after stroke may determine if the response promotes neuronal healing or damage. The field is still young, but Kasper wonders if it might be possible to use gut microbes’ immune-modulating capacities to enhance regulatory responses rather than suppress total immunity. This is especially needed for MS treatment, where most current drugs block immune activity wholesale. “We should be using agents that drive a positive response,” he says. “If we can drive the immune system from the gut upward and do it in a positive way, I think that will give rise to greater benefit and far less side effects than [current drugs].” |
References
- J. Kipnis et al., “T cell deficiency leads to cognitive dysfunction: Implications for therapeutic vaccination for schizophrenia and other psychiatric conditions,” PNAS, 101:8180-85, 2004.
- N.C. Derecki et al., “Regulation of learning and memory by meningeal immunity: A key role for IL-4,” J Exp Med, 207:1067-80, 2010.
- O. Rapalino et al., “Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats,” Nat Med, 4:814-21, 1998.
- A.L. Hawthorne, P.G. Popovich, “Emerging concepts in myeloid cell biology after spinal cord injury,” Neurotherapeutics, 8:252-61, 2011.
- D.M. McTigue et al., “Localization of transforming growth factor-beta1 and receptor mRNA after experimental spinal cord injury,” Exp Neurol, 163:220-30, 2000.
- R. Shechter et al., “Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice,” PLOS Med, 6:e1000113, 2009.
- K.A. Kigerl et al., “Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord,” J Neurosci, 29:13435-44, 2009.
- P. Edwards et al., “Final results of MRC CRASH, a randomized placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months,” Lancet, 365:1957-59, 2005.
- K. Baruch et al., “CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging,” PNAS, 110:2264-69, 2013.
- G. Kunis et al., “IFN-γ-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair,” Brain, 136:3427-40, 2013.
- J.T. Walsh et al., “MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4,” J Clin Invest, 125:699-714, 2015.
- T.L. Roth, et al., “Transcranial amelioration of inflammation and cell death following brain injury,” Nature, 505:223-28, 2014.
- A.R. Simard et al., “Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease,” Neuron, 49:489-502, 2006.
- J-P. Michaud et al., “Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta,” Cell Reports, 5:646-53, 2013.
- J-P. Michaud et al., “Hematopoietic MyD88-adaptor protein acts as a natural defense mechanism for cognitive deficits in Alzheimer’s disease,” Stem Cell Rev, 8:898-904, 2012.
- A. Lampron et al., “Inefficient clearance of myelin debris by microglia impairs remyelinating processes,” J Exp Med, 212:481-95, 2015.
- K. Baruch et al., “Breaking immune tolerance by targeting Foxp3+ regulatory T cells mitigates Alzheimer’s disease pathology,” Nat Commun, 6:7967, 2015.
- K. Baruch et al., “PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease,” Nat Med, 22:135-37, 2016.
- S. Hong et al., “Complement and microglia mediate early synapse loss in Alzheimer mouse models,” Science, 352:712-16, 2016
- A. Brynskikh et al., “Adaptive immunity affects learning behavior in mice,” Brain Behav Immun, 22:861-69, 2008.
- A.J. Filiano et al., “Unexpected role of interferon-γ in regulating neuronal connectivity and social behavior,” Nature, 535:425-29, 2016.
- K.M. Nautiyal et al., “Serotonin of mast cell origin contributes to hippocampal function,” Eur J Neurosci, 36:2347-59, 2012.
- K.M. Nautiyal et al., “Brain mast cells link the immune system to anxiety-like behavior,” PNAS, 105:18053-57, 2008.
- S. Asadi, T.C. Theoharides, “Corticotropin-releasing hormone and extracellular mitochondria augment IgE-stimulated human mast-cell vascular endothelial growth factor release, which is inhibited by luteolin,” J Neuroinfl, 9:85, 2012.
Clarification (November 2): This story has been updated to clarify that Kipnis has worked with collaborators outside of his own group on his work that identified social differences in mice lacking B and T cells. The Scientist regrets any confusion.
Interested in reading more?